The Diverse Yet Crucial Battlefields: Electrolysis vs Galvanic Corrosion
Introduction
Every marine enthusiast understands the inherent challenges in maintaining the integrity of their beloved vessels. One particular area of concern is corrosion, a persistent adversary that can jeopardize both the aesthetic and functional quality of your boat. Two primary culprits—electrolysis and galvanic corrosion—can be confusing due to their seemingly similar mechanisms, but it's crucial to differentiate between them. Understanding these processes is the first step towards securing your marine investment against these destructive forces.
Electrolysis and Galvanic Corrosion: A Common Enemy, Yet Distinct Foes
At the surface, electrolysis and galvanic corrosion may appear similar—they both involve metallic degradation in the presence of an electrolyte. However, they fundamentally differ in the nature of their underlying mechanisms, which subsequently dictates the approach to prevention and treatment.
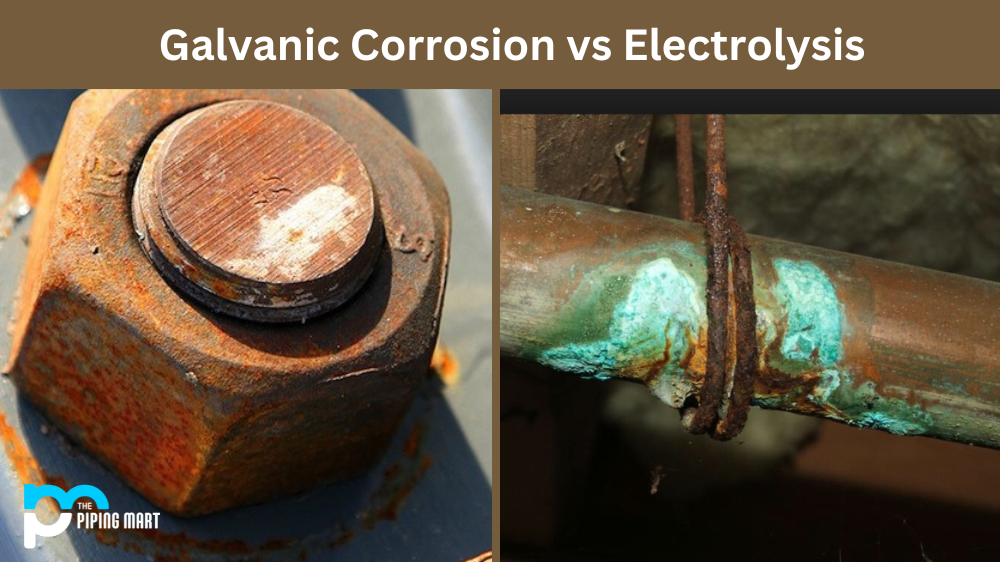
Electrolysis
Electrolysis is a process triggered by stray electrical current finding its path into the metal parts of your boat, leading to rapid corrosion. This current can be sourced from your own boat's electrical system or external sources, such as faulty dockside power sources or neighbouring vessels. The primary challenge with electrolysis is that it's often unpredictable, accelerated, and may cause substantial damage if left unchecked.
Galvanic Corrosion
On the other hand, galvanic corrosion occurs when two dissimilar metals come into contact within an electrolyte, such as seawater. The less noble metal (anode) sacrifices itself by corroding and protecting the more noble metal (cathode). For instance, in a marine setting, if bronze and aluminium are in direct contact and surrounded by seawater, the aluminium will corrode to protect the bronze. This form of corrosion is predictable and slow but can cause long-term damage if not appropriately managed.
Protection is Paramount: Ingot's Role
Protection against these corrosive processes is paramount. Ingot, a renowned provider of quality marine equipment, offers a comprehensive range of protection systems to ensure the safety and longevity of your vessel.
Anodes, which act sacrificially to protect your valuable metallic parts, are a key asset in the fight against galvanic corrosion. By installing an anode of a less noble metal, like zinc or magnesium, you can control and confine the corrosive process to the anode, safeguarding your critical boat components.
When it comes to electrolysis, a well-designed and implemented earthing system, like our earthing arm straps, can help ground stray currents and prevent electrolysis. Ensuring your vessel’s electrical system is in top shape is also essential.
Conclusion
The fight against electrolysis and galvanic corrosion is ongoing, but understanding the distinction between the two will enable you to effectively address each. With a keen eye and the right equipment, like those provided by Ingot, you can keep your marine assets protected and extend their lifespan. Remember, regular maintenance is key and will go a long way in preserving your marine investments.
Make your choice for reliable anodes and earthing arm straps today and journey the seas with assurance.



Comments